Universal Chalcidoidea Database
Collecting and preserving chalcidoids
Mailing dry specimens to specialists for identification
Material sent to specialists for identification must include sufficient data, ie at least country, locality, date, host (if reared) and collector. Do not sent specimens that have no data or only reference numbers as these might not be examined and may be disposed of.
Storage
Always store material (particularly that in alcohol) in a cool, dark place. Material in alcohol is best stored in a deep-freeze and NEVER in direct sunlight or on open shelves or bench tops.
Preparation of material
a) Larger chalcidoids (>2.5mm in length). These can be adequately preserved in clean 70% alcohol and stored in suitably sized leak-proof containers. Preferably specimens should be killed by being placed directly into the alcohol - this ensures that the wings are held in an ideal position for subsequent examination. If material is to be sent through the post it is best to hold the specimens in position with some tightly compressed cotton wool (Fig. 7, below). All air bubbles must be expelled from the section of the tube containing the specimens and a label containing the data should be placed in the other section. Any air bubbles or labels in the section containing specimens may move around violently during transit and damage the specimens.
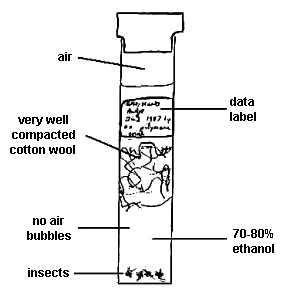 |
| Fig. 7 Method for storing microhymenoptera in vials to prevent breakage during transport. |
b) Smaller chalcidoids (<2.5mm length). This method applies especially to Encyrtidae, Aphelinidae and Trichogrammatidae. Where a long series is available (>20 specimens) kill half in an atmosphere of ethyl acetate and store dry in gelatin capsules (Fig. 8, below). Any material that has died naturally should also be stored dry in gelatin capsules, not in alcohol. Other material should be killed directly in 70% alcohol. For mailing, these can be held in a suitable small glass vial or Eppendorf vial packed in a similar manner to larger specimens (as in Fig. 7, above).
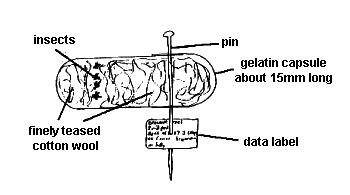 |
|
Fig. 8 Storing microhymenoptera in a gelatin capsule
|
In this case it is extremely important to compress the cotton wool as firmly as possible to prevent the smaller insects becoming entangled in it. To store dry in a gelatin capsule firstly tease a very small quantity of good quality cotton wool until it is fine and wispy. Put this into the bottom of a gelatin capsule. Next place the specimens in top of the cotton wool (up to 30 smaller (<1mm long) or 10 larger (>1mm long) specimens) then push some more finely teased cotton wool onto these to hold them firmly in place. This is best done when the specimens are freshly killed and under a dissecting microscope to prevent damage to the specimens which may render them useless for taxonomic study, eg loss of antennae or wings. Push on the top of the capsule and hold in place by pushing a pin through its inner and outer parts. Finally attach a label giving minimum data as above.
IMPORTANT: In areas of high humidity store in an air-conditioned room or in an airtight box containing a crystal or two of thymol to prevent fungal growth on the specimens.
Mailing material
Put material in a strong cardboard box and hold firmly in place by using cotton wool, tissue paper or (in the case of dry card-mounted material) by cross-pinning. Place this container in a second, larger strong container which contains shock-absorbent material such as expanded polystyrene chips, wood wool or even tightly rolled newspaper (see Fig. 9). Gelatin capsules containing specimens as described above can be placed in wells cut into a suitably sized, 1.5cm thick piece expanded polystyrene and posted in an envelope.
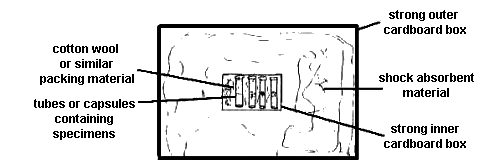 |
| Fig. 9 Suitable method for mailing microhymenoptera |
Last updated 08-Jun-2004 Dr B R Pitkin