Universal Chalcidoidea Database
Collecting and preserving chalcidoids
Storing and preserving
(1) Layering
This is probably the best method of storing dry material if large numbers of insects are involved but only if they are still flexible. This is achieved by layering insects between sheets of Cellosene® wadding, soft toilet paper, tissue handkerchiefs, etc. It is best to layer material in an airtight plastic container. Place a few sheets of Cellosene® wadding in the bottom of the container, sprinkle insects thinly on these, place another few sheets of wadding on top and the press down firmly. Continue by placing a thin sheet of paper on top followed by another few sheets of wadding and then insects and so on until the box is almost full. Put some well teased cotton wool on top to hold the layers in place plus one or two small crystals of thymol to prevent fungal growth. Place the lid on the box and seal with adhesive tape. Inserting paper between consecutive layers prevents the layers from sticking together. So not put a single layer of wadding between two layers of insects as his leads to insects sticking to the lower surface of the wadding. These become damaged or lost when the layer is removed to examine specimens on the upper surface. After a few days, insects preserved in this way will become dry and easily damaged. Therefore it will be necessary to soften them before examination by putting the whole box in suitable relaxing chamber to soften the insects (se "Relaxing material"). Experience has shown that subsequent examination is facilitated if the material is stored in boxes measuring about 5x4x2cm.
(2) Storing in glass or plastic tubes
This is probably the quickest method for large number of specimens. Material can be stored in rolled up pieces of tissue in 2.5cm or 1.2cm diameter tubes. This is best done by pushing pieces of tissue paper into the top of the tube using a pencil to form a well. Pour the specimens into this well, using a paper funnel if necessary. If material is fresh or relaxed and is to be sent through the post, gently roll the top of the tissue between the thumb and index finger, just above the specimens until the insects are held firmly inside. Then cut the top off the tissue and drop it into the tube and add a crystal of thymol to prevent mould, and finally stopper the tube. This is an ideal method of storing fewer than 300 specimens (2 tissue 'balls' in one tube). It can also be used for unmounted, critical point dried material, but in this case it is best not to roll the tissue but just to cut it around the rim of the tube and push it into the bottom of the tube. To relax the specimens prior to examination, remove the stopper and place the whole tube in a relaxing box (see "Relaxing specimens"). The cotton wool plug and tissue 'ball' can then be removed with no risk of damage to the enclosed insects. After examination the material can be stored in the same tissue
'ball' as before. When insects are stored loose in glass, or plastic tubes, it is tempting to examine them in the tube by 'rolling' it to get a better view of the specimens. This may cause considerable damage. Storing insects in a tissue 'ball' also prevents them from sticking to the sides of the tube and allows thymol crystals to be added to prevent fungal growth.
(3) Storing in gelatin capsules
Up to 10 specimens of larger species (<1mm in length) can be stored dry in a single capsule (Fig. 4). Tease out some good quality cotton wool until it is very fine and wispy and pull off a small piece and gently push into the bottom part of a 15-2- mm gelatin capsule without excessively compressing the cotton wool. Place specimens onto cotton wool in bottom of capsule and, viewing through a stereomicroscope, gently push a second, small piece of finely teased cotton wool into the capsule, thus holding specimens in place. Take care not to push too hard or legs and antennae may be broken. The specimens should now be held firmly in place. Gently slide the cap of the gelatin capsule onto the base and hold in place by pushing an entomological pin through the cap so that it also passes through the base of the capsule. Push the pin through a data label.
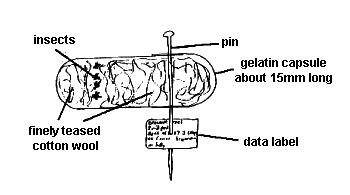 |
| Fig. 4 Storing microhymenoptera in a gelatin capsule. |
If there is a risk of material being contaminated by fungi, such as in areas of high humidity and temperature, then capsules should be stored in a airtight container containing a few thymol crystals to prevent fungal growth.
(4) Storing in alcohol
Alcohol is a very good medium in which to store small insects. It facilitates their future examination and can also preserve genetic material that may be useful for other studies. Unfortunately small insects deteriorate rapidly if stored in alcohol in unsuitable conditions, such s relatively high temperatures or in bright daylight. Even highly metallic, dark bodied insects can lose all colour and take on an orange hue with two weeks if stored in daylight and at temperatures in excess of 30°C. It is best to maintain alcohol collections in the dark and at a low temperature. A deep-freeze run at -30°C is ideal as this will also preserve genetic material more reliably.
Last updated 07-Jun-2004 Dr B R Pitkin